At a glance
Vaccination coverage data for pregnant women are obtained weekly from the Vaccine Safety Datalink (VSD) during the respiratory illness season and displayed on the RespVaxView dashboard. At the end of each respiratory illness season, a post-season estimation methodology is used that allows more time to capture vaccine administrations, which may yield vaccination coverage estimates that are higher. Both weekly and post-season estimation methodologies provide useful data to help understand vaccination coverage estimates among pregnant women.
Summary
The Vaccine Safety Datalink (VSD) is a collaborative project between CDC’s Immunization Safety Office and 13 integrated health care organizations in eight U.S. states; 11 sites provide data and 2 additional sites provide subject matter expertise (1). Among the 11 sites providing data, the VSD population includes approximately 15 million persons, including roughly 3 million women aged 18–49 years. To monitor vaccination coverage and safety, CDC obtains influenza, COVID-19, and respiratory syncytial virus (RSV) vaccination data from the VSD sites’ electronic health records, health insurance claims, and linked state and/or regional Immunization information systems (IIS).
Influenza, COVID-19, and RSV vaccination coverage data for pregnant women are obtained weekly during the respiratory illness season and displayed on the RespVaxView dashboard. A weekly estimation methodology is used to obtain these rapid vaccination coverage estimates during the season. At the end of each respiratory illness season, a post-season estimation methodology is used that allows more time to capture vaccine administrations from electronic health records and applies a continuous VSD enrollment criterion, which may yield higher estimates of vaccination coverage. Using weekly estimation methodology, coverage estimates are available in near real time, during the current season. However, post-season estimation methodology allows time for complete capture of all pregnancies in the study period and for rectifying data lags in the capture of vaccination and health care utilization data and for the inclusion of additional data on patient demographics, health conditions, and pregnancy trimester information. Assessing vaccination coverage using both methodologies can help derive a more complete picture of influenza, COVID-19, and RSV vaccination coverage among pregnant women.
Methods
A dynamic pregnancy algorithm based on International Classification of Diseases, Tenth Edition (ICD-10) diagnosis codes, procedure codes, estimated dates of delivery, and last menstrual period dates from electronic health records is used to identify pregnancies weekly at 10 participating, data-contributing VSD sites. Because the algorithm identifies pregnancies based on coded health care utilization data, pregnancies are generally identified at approximately 10 weeks’ gestation (1).
Weekly estimation methodology for influenza, COVID-19, and RSV includes women aged 18–49 years with a pregnancy during the current respiratory illness season through the respective week of interest. Vaccination coverage is cumulative through the current week; pregnant women are not removed from the numerator or denominator when the pregnancy ends.
Post-season estimation methodology includes women who are pregnant during the current season and continuously enrolled within a VSD health care system during the vaccination period, as defined below. The continuous enrollment criterion may increase the completeness of vaccination data capture. However, VSD incorporates state IIS data that capture vaccination information during periods of non-enrollment. Women are considered "enrolled" in the VSD population for a given month if they are covered members of their health care system for that entire month. Additional site-specific criteria may be applied, such as living in a geographic service area, and/or attaining a minimal level of health care utilization. At one VSD site, any record of health care utilization over the past 18 months is used as a substitute for current-month health system membership. Additionally, post-season estimation methodology, for all three vaccine types, excludes pregnancies that ended in spontaneous or induced abortions, or that ended prior to 14 weeks’ gestation. Post-season estimation methodology for influenza and COVID-19 includes women aged 18–49 years. For RSV vaccination coverage, the methodology includes women aged 12–55 years due to aggregate data received from the VSD sites, although RSV-vaccinated women aged 12–17 years and 50–55 years comprise a small proportion of all those included in the analysis (N=217).
Influenza
Weekly Estimation Methodology
Weekly estimation methodology for influenza vaccination coverage included women aged 18–49 years who were pregnant at least one day between August 1 and each week of interest through March 31 of the respective respiratory illness season.
Post-season Estimation Methodology
Coverage estimates were calculated as above with the additional criterion that pregnant women were continuously enrolled in a VSD health care system during the August─March analytic period. Vaccinations that occurred between July 1 and March 31 of the respective season before or during pregnancy were included in the analysis for both weekly and post-season estimation methodologies.
Comparison of Weekly and Post-season Estimates
Overall, influenza vaccination coverage using post-season estimation methodology has been higher than influenza vaccination coverage using weekly estimation methodology for all seasons from 2019─2020 through 2023─2024. Post-season influenza vaccination coverage was 43.8% at the end of the 2023─2024 season, compared with 38.1% for weekly estimation methodology (Figure 1). Vaccination coverage by race and ethnicity using weekly estimation methodology is reported on the Weekly Flu dashboard.
Figure 1. Influenza vaccination coverage* among pregnant women† weekly estimation methodology§ vs. post-season estimation methodology¶ estimates, 2019─2020 through 2023─2024 respiratory illness seasons – Vaccine Safety Datalink
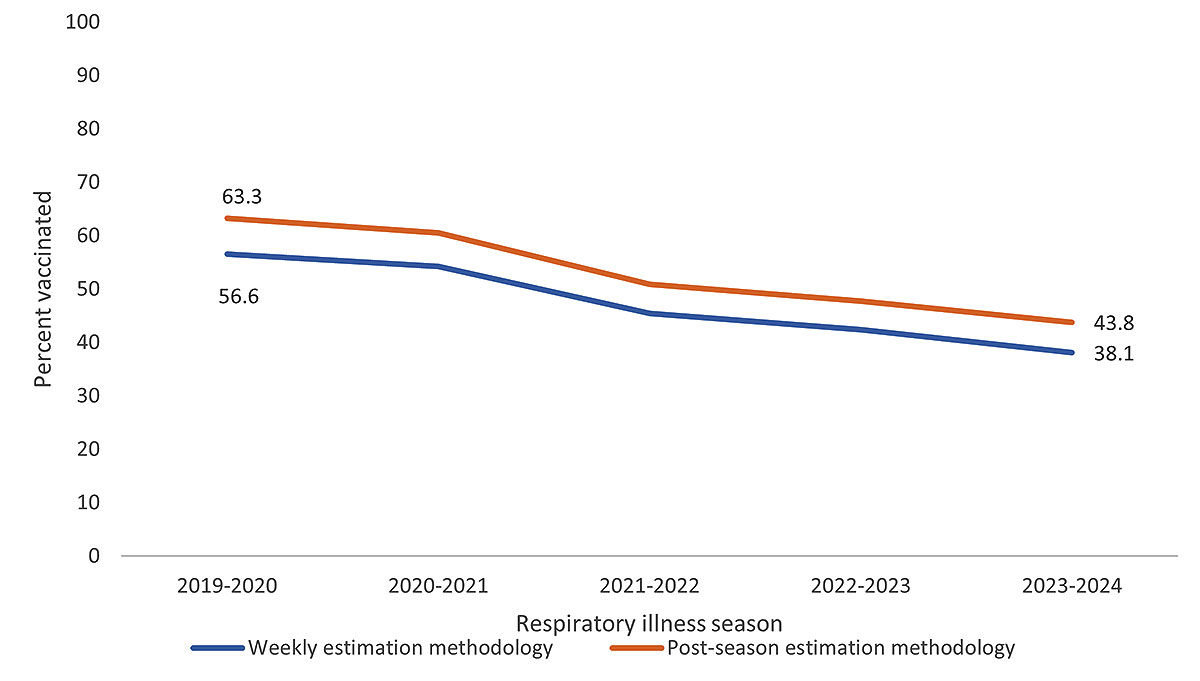
*Receipt of influenza vaccination from July-March of the respective respiratory illness season.
†All pregnant women identified in the VSD during August 1 to March 31 of the respective seasons. Estimates include vaccinations received before or during pregnancy.
§In weekly estimation methodology, pregnant women are not removed from the numerator or denominator when the pregnancy ends. No requirements for length of enrollment in a VSD site are applied.
¶Post-season estimation methodology for influenza vaccination coverage includes women pregnant at least one day from August 1 to March 31 of the respective season, and continuously enrolled in a VSD health care system during that period.
COVID-19
Weekly Estimation Methodology
The 2023─2024 COVID-19 vaccine was recommended starting September 14, 2023. Weekly estimation methodology to assess COVID-19 vaccination coverage included women aged 18–49 years and pregnant at least one day starting September 26, 2023 (Weekly COVID dashboard).
Post-season Estimation Methodology
Coverage estimates were calculated as above with the additional criterion that pregnant women were continuously enrolled in a VSD health care system during pregnancy.
Vaccinations that occurred between September 14, 2023, and August 21, 2024, before or during pregnancy, were used for the weekly estimation methodology; vaccinations that occurred between September 26, 2023, and August 21, 2024, before or during pregnancy, were used for the post-season estimation methodology.
Comparison of Weekly and Post-season Estimates
COVID-19 vaccination coverage using post-season estimation methodology overall was 1.1 percentage points higher than vaccination coverage using weekly estimation methodology. By race and ethnicity, post-season methodology estimates were higher than weekly methodology estimates in all race and ethnicity groups, ranging from 0.5-2.4 percentage points higher (Figure 2).
Figure 2. Receipt of 2023─2024 COVID-19 vaccination among pregnant women* weekly estimation methodology† vs. post-season estimation methodology§ estimates by race and ethnicity – Vaccine Safety Datalink
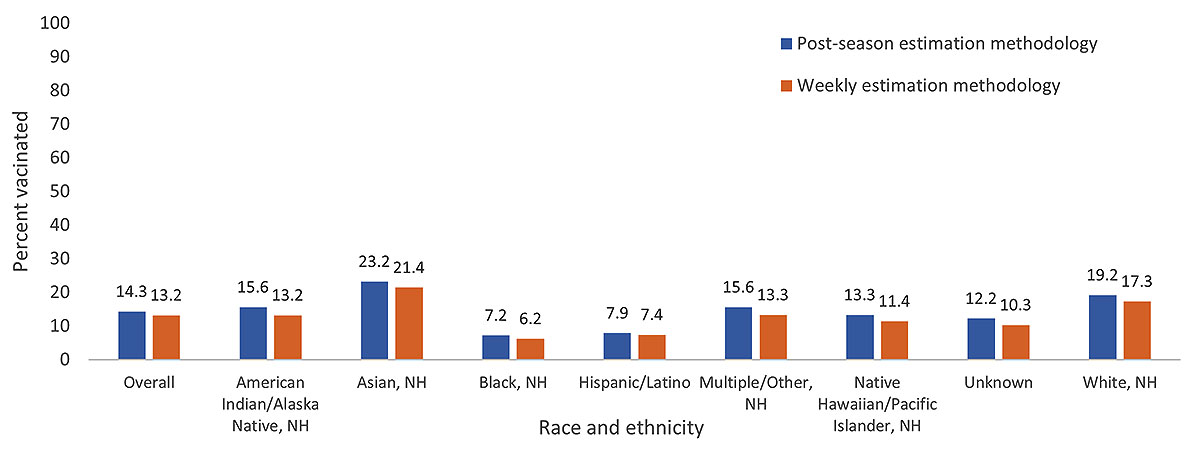
Abbreviations: NH=Non-Hispanic
*All pregnant women identified in the VSD from September 26, 2023─August 21, 2024. Estimates include vaccinations received before or during pregnancy.
†In weekly estimation methodology, pregnant women are not removed from the numerator or denominator when the pregnancy ends. No requirements for length of enrollment in a VSD site are applied. Vaccinations from September 12, 2023─August 21, 2024, were included in the analysis.
§Post-season estimation methodology for the 2023─24 COVID-19 vaccination coverage includes women pregnant at least one day from September 26, 2023─August 21, 2024, and continuously enrolled in a VSD healthcare system during pregnancy. Vaccinations from September 26, 2023─August 21, 2024, were included in the analysis.
RSV
Weekly Estimation Methodology
RSV vaccination for pregnant women was first recommended on September 22, 2023 (2). To assess RSV vaccination coverage using weekly estimation methodology, pregnant women aged 18–49 years who were at least 32 weeks' gestation since September 22, 2023, were included; the denominators may have also included pregnant women who were past the recommended vaccination window of 32-36 weeks' gestation before September 22, 2023. Implementation timelines and availability of RSV vaccine differed across participating VSD sites; therefore, for weekly estimation methodology, early coverage estimates may have included women in the denominator who were not able to receive vaccination within their health systems starting September 22, 2023, when the vaccine was first recommended. By November 25, 2023, all VSD sites were offering RSV vaccination to pregnant women (Weekly RSV dashboard).
Post-season Estimation Methodology
Post-season estimation methodology for RSV vaccination coverage included all women aged 12–55 years who were 32-36 weeks' gestation, pregnant at least one day between September 22, 2023, and January 31, 2024, and had a live birth outcome between September 22, 2023, and March 31, 2024. Women who were more than 36 weeks gestation as of September 22, 2023, were excluded from the analysis. Eligible pregnant women were continuously enrolled in their VSD health system throughout the pregnancy.
For both the weekly and post-season estimation methodologies, vaccinations that occurred between September 22, 2023, and January 31, 2024, were included in the analysis.
Comparison of Weekly and Post-season Estimates
RSV vaccination coverage through January 31, 2024, was 30.5% using post-season estimation methodology, compared with 17.8% using weekly estimation methodology. By race and ethnicity, post-season estimates were higher for all groups when compared with weekly methodology estimates. Similar to estimates using weekly methodology, RSV vaccination coverage using post-season estimation methodology was highest among non-Hispanic, Asian pregnant women (40.3%) and lowest among non-Hispanic, Black pregnant women (23.3%) (Figure 3).
Figure 3. Respiratory Syncytial Virus (RSV) vaccination coverage* among pregnant women† weekly estimation methodology§ vs. post-season estimation methodology¶, 2023─2024 respiratory illness season, by race and ethnicity – Vaccine Safety Datalink
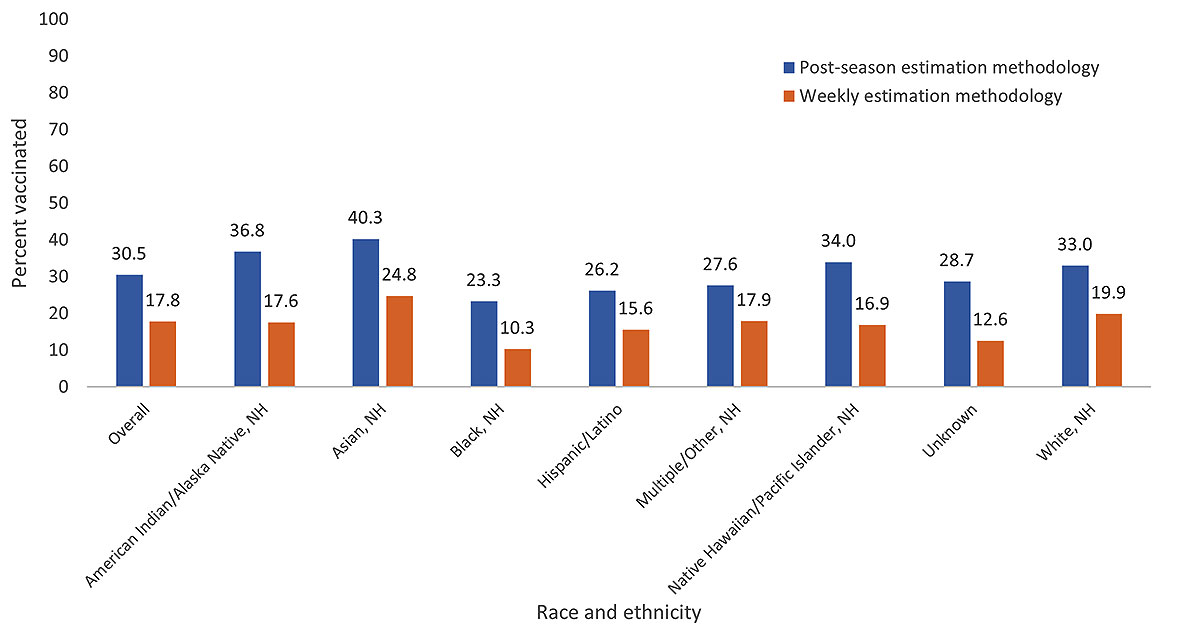
Abbreviations: NH=Non-Hispanic; RSV=Respiratory syncytial virus
*Receipt of RSV vaccination from September 22, 2023─January 31, 2024.
†All pregnant women identified in the VSD from September 22, 2023─January 31, 2024.
§ In weekly estimation methodology, the denominator includes those who reached at least 32 weeks' gestation since September 22, 2023. The denominator may also include pregnant women who were past the recommended vaccination window of 32-36 weeks' gestation before September 22, 2023. Pregnant women are not removed from the numerator or denominator when the pregnancy ends. No requirements for length of enrollment in a VSD site are applied, and vaccinations given outside of a VSD site may not be included. Vaccinations from September 22, 2023─January 31, 2024, were included in the analysis. These estimates only include women 18–49 years.
¶Post-season estimation methodology for RSV vaccination coverage includes women aged 12─55 years who were pregnant and at least 32-36 weeks gestation, continuously enrolled in a VSD healthcare system during pregnancy and during the period September 22, 2023–January 31, 2024, and had a live birth outcome from September 22, 2024─March 31, 2024. Vaccinations from September 22, 2023─January 31, 2024, were also included in the analysis.
Limitations
The findings in this report are subject to at least three limitations. First, the findings from the weekly and post-season estimation methodologies may not be generalizable to all pregnant women in the United States. Second, vaccinations administered outside of the VSD health care system may not be included. Finally, applying the dynamic pregnancy algorithm might result in some misclassification of pregnancy status and dates, especially in the weekly estimation methodology when data from ongoing pregnancies might be incomplete.
Conclusion
Both weekly and post-season estimation methodologies provide useful data to help understand vaccination coverage estimates among pregnant women, although there are strengths and limitations to both methodologies (Table 1). Estimates using weekly estimation methodology may yield lower vaccination coverage, substantially so for RSV; however, they provide critical information for timely decision making given they provide rapid insight into vaccination uptake weekly during the season. Estimates using post-season estimation methodology become available after the respiratory illness season ends and provide a more robust estimate of vaccination coverage for the season. The enrollment criteria applied to post-season estimation methodology may also result in higher estimates. This is because women who are continuously enrolled tend to have higher coverage, with better data capture, than women who may have gaps in enrollment. The vaccination coverage estimates using the post-season methodology are comparable to vaccination coverage estimates among pregnant women using the Internet panel survey, which assess coverage at the end of each respiratory illness season (3). Assessing vaccination coverage estimates at different time points throughout the respiratory illness season may help implement certain actionable items such as provider recommendations for vaccination and education for pregnant women on the safety and efficacy of these vaccines, which may also help increase vaccination coverage (3).
Authors: Mehreen Meghani, MPH1, Hilda Razzaghi, PhD1, Allison L. Naleway, PhD2, Bradley Crane, MS2, Stephanie A. Irving, MHS2, Carla L. Black, PhD1
Acknowledgments: Vaccine Safety Datalink investigators, project managers, and data managers.
1Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; 2Center for Health Research, Kaiser Permanente Northwest, Portland, Oregon
References
- Naleway AL, Crane B, Irving SA, et al. Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination. Therapeutic Advances in Drug Safety. June 2021. doi: 1177/20420986211021233
- Fleming-Dutra KE, Jones JM, Roper LE, et al. Use of the Pfizer Respiratory Syncytial Virus Vaccine During Pregnancy for the Prevention of Respiratory Syncytial Virus–Associated Lower Respiratory Tract Disease in Infants: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–1122. doi: http://dx.doi.org/10.15585/mmwr.mm7241e1
- Kahn KE, Garacci E, Razzaghi H, et al. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women—United States, April 2024. Atlanta, GA: US Department of Health and Human Services, CDC; 2024. https://www.cdc.gov/fluvaxview/coverage-by-season/pregnant-april-2024.html
Appendix
Table 1. Differences in timing and enrollment criteria and impacts on coverage estimates between weekly in-season and post-season estimation methodologies for influenza, COVID-19, and Respiratory Syncytial Virus (RSV) vaccines among pregnant women, Vaccine Safety Datalink
| Timing | Enrollment Criteria | Possible Impacts on Coverage Estimates | |
|---|---|---|---|
| Weekly Estimation Methodology | Pros: Coverage estimates available in near real time, during the current season. Internally administered health system vaccination data is often available almost immediately. Most pregnancies are identified within 10-12 weeks after start of pregnancy.
Cons: Pregnancies starting at the end of the respiratory illness season are less likely to be identified than pregnancies earlier in study period.
|
Pros: Does not limit population to those with continuous healthcare system enrollment. The resulting population may better represent general population.
Cons: No health system enrollment criteria. Pregnancy and vaccination data may be less complete for those not continuously enrolled.
|
Weekly estimates may change as data matures. Both pregnancy and vaccination data can have time lags associated with them. The subset without continuous enrollment may have less access to care and relatively lower vaccination coverage. Vaccine data capture may be less complete for the subset not continuously enrolled. However, the availability of immunization information system data improves data completeness. |
| Post-season Estimation Methodology | Pros: Allows time for complete capture of all pregnancies in the study period. Rectifies data lags in the capture of vaccination and health care utilization data, especially from outside of healthcare system.
Cons: Does not provide information during the current season. Estimates are usually not available until 4 to 6 months after the end of the study period.
|
Pros: Continuous enrollment during pregnancy and/or during respiratory illness season, increasing completeness of data capture.
Cons: May result in a less representative sample, selecting individuals with more access to health care and thus potentially more likely to be vaccinated.
|
Because the post-season methodology requires continuous enrollment, the resulting population likely has higher vaccination coverage than those without. Vaccination data capture may also be better for the continuously enrolled. |